Therapeutic Areas
Novel modalities and therapeutics have changed the landscape of biopharma in recent years. This surge in complex, engineered therapeutics appears set to continue. Bi-, tri- and multi-specifics are showing potential to improve ORR, while CAR-T therapies move us towards personalized cancer treatment. Cell and Gene therapies promise life saving treatments for rare diseases that cannot be treated by conventional modes of treatment.
Each therapy class has its own unique challenges – but some are common to them all. They all have complex MoA and yet only limited data is available to understand PK-PD relationship. Further, each has unique and multi factorial interactions with physiology. Take ADCs for example, factors in consideration include payload potency, bystander effect, Drug-antibody ratio, linker stability, and the effects of all of these aspects on efficacy and safety.
The twin challenge of low data & physiological complexity is precisely what QSP can handle. We have steadily developed our base of experience to address questions of interest in all of the above area.
Key Questions that M&S can address:
Cell Rx:
- What is the adequate dose for the cell therapy to treat rare neurological disorder?
LNP therapeutic:
- What is the optimal design for LNP to maximize the bioavailability at target site?
- What is the optimal dose for LNP-based cancer vaccine?
Bispecific:
- Explain the relationship between trimer formation (binding affinity, avidity) and the observed efficacy/safety for a bi-specific antibody.
- What is the ideal dose fractionation regimen for improving therapeutic window?
ADC:
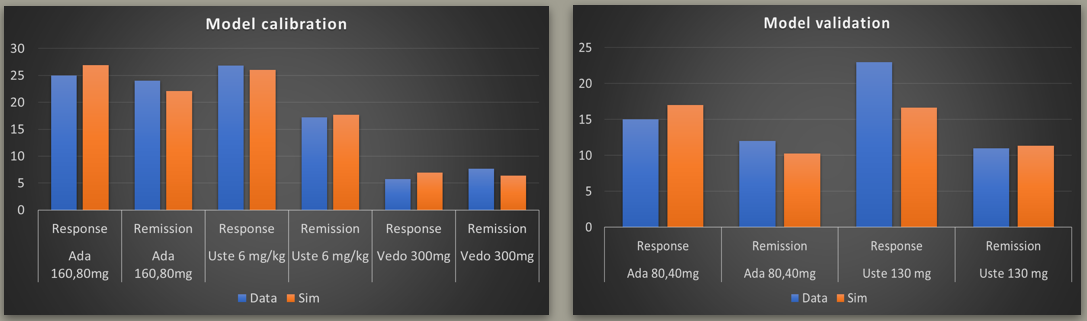
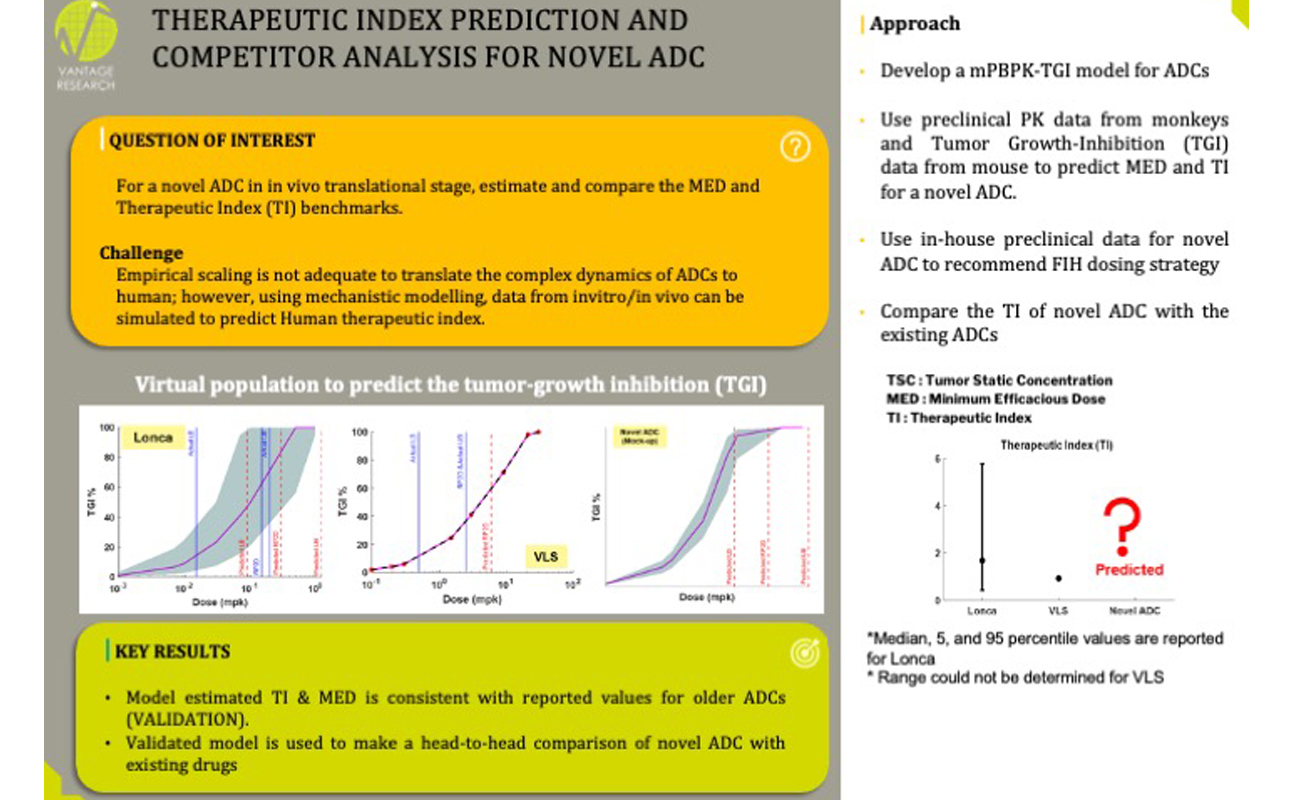
- What should be FIH dose range for a novel ADC? What is the Minimum efficacious dose (MED)?
- What would be the expected Phase 3 RECIST Objective Response Rate (ORR)?
- What would be the expected Phase 3 efficacy of ADC in combination with checkpoint inhibitor?